Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yoshida, Naoki; Ono, Takuya; Yoshida, Ryoichiro; Amano, Yuki; Abe, Hitoshi
Journal of Nuclear Science and Technology, 57(11), p.1256 - 1264, 2020/11
Times Cited Count:8 Percentile:71.58(Nuclear Science & Technology)Emphasis has been placed on the behavior of ruthenium (Ru) in the evaporation to dryness accident due to the loss of cooling functions (EDLCF) of high-level liquid waste in fuel reprocessing plants. It is because Ru would form volatile compounds such as ruthenium tetroxide (RuO ) and could be released into the environment with other coexisting gasses such as nitric acid (HNO
) and could be released into the environment with other coexisting gasses such as nitric acid (HNO ), water (H
), water (H O). To contribute to the safety evaluation of this accident, we experimentally evaluated the decomposition and chemical change behavior of the gaseous RuO
O). To contribute to the safety evaluation of this accident, we experimentally evaluated the decomposition and chemical change behavior of the gaseous RuO (RuO
(RuO (g)) under the various atmospheric conditions: temperature and composition of coexisting gasses. As a result, the behavior of the RuO
(g)) under the various atmospheric conditions: temperature and composition of coexisting gasses. As a result, the behavior of the RuO (g) was diverse depending on the atmospheric conditions. In the experiments with the dry air or H
(g) was diverse depending on the atmospheric conditions. In the experiments with the dry air or H O vapor, decomposition of RuO
O vapor, decomposition of RuO (g) was observed. In the experiment with the mixed gas which containing HNO
(g) was observed. In the experiment with the mixed gas which containing HNO , almost no decomposition of the RuO
, almost no decomposition of the RuO (g) was observed, and chemical form of the RuO
(g) was observed, and chemical form of the RuO (g) was retained.
(g) was retained.
 O)
O) ]
] with nitrate ions by using density functional theory calculation
with nitrate ions by using density functional theory calculationKato, Akane*; Kaneko, Masashi; Nakashima, Satoru*
RSC Advances (Internet), 10(41), p.24434 - 24443, 2020/06
Times Cited Count:6 Percentile:31.74(Chemistry, Multidisciplinary)Complexation reactions of ruthenium-nitrosyl complexes in HNO solution were investigated by density functional theory (DFT) calculations in order to predict the stability of Ru species in high-level radioactive liquid waste (HLLW) solution. Equilibrium structure of [Ru(NO)(NO
solution were investigated by density functional theory (DFT) calculations in order to predict the stability of Ru species in high-level radioactive liquid waste (HLLW) solution. Equilibrium structure of [Ru(NO)(NO )
) (H
(H O)
O) ] obtained by DFT calculations reproduced the experimental Ru-ligands bond lengths and IR frequencies reported previously. Comparison of the Gibbs energies among the geometrical isomers revealed that the complexation reactions of the ruthenium-nitrosyl complexes with NO
] obtained by DFT calculations reproduced the experimental Ru-ligands bond lengths and IR frequencies reported previously. Comparison of the Gibbs energies among the geometrical isomers revealed that the complexation reactions of the ruthenium-nitrosyl complexes with NO
 proceed via the NO
proceed via the NO
 coordination to the equatorial plane toward the Ru-NO axis. We also estimated Gibbs energy differences on the stepwise complexation reactions to succeed in reproducing the fraction of Ru-NO species in 6 M HNO
coordination to the equatorial plane toward the Ru-NO axis. We also estimated Gibbs energy differences on the stepwise complexation reactions to succeed in reproducing the fraction of Ru-NO species in 6 M HNO solution, such as in HLLW, by considering the association energy between the Ru-NO species and the substituting ligands. Electron density analyses of the complexes indicated that the strength of the Ru-ligands coordination bonds depends on the stability of the Ru species and the Ru complex without NO
solution, such as in HLLW, by considering the association energy between the Ru-NO species and the substituting ligands. Electron density analyses of the complexes indicated that the strength of the Ru-ligands coordination bonds depends on the stability of the Ru species and the Ru complex without NO
 at the axial position is more stable than that wit NO
at the axial position is more stable than that wit NO
 , which might attribute to the difference in the trans influence between H
, which might attribute to the difference in the trans influence between H O and NO
O and NO
 . Finally, we demonstrated the complexation kinetics in the reactions
. Finally, we demonstrated the complexation kinetics in the reactions 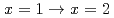 . The present study is expected to enable us to model the precise complexation reactions of platinum-group metals in HNO
. The present study is expected to enable us to model the precise complexation reactions of platinum-group metals in HNO solution.
solution.
 Ru M
Ru M ssbauer parameters of ruthenium-nitrosyl complexes
ssbauer parameters of ruthenium-nitrosyl complexesKaneko, Masashi; Kato, Akane*; Nakashima, Satoru*; Kitatsuji, Yoshihiro
Inorganic Chemistry, 58(20), p.14024 - 14033, 2019/10
Times Cited Count:12 Percentile:63.71(Chemistry, Inorganic & Nuclear)We applied density functional theory calculations to ruthenium-nitrosyl complexes, which are known to exist in high-level radioactive waste, to give a theoretical correlation between  Ru M
Ru M ssbauer spectroscopic parameters (
ssbauer spectroscopic parameters ( and
and 
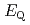 ) and ligand field strength (
) and ligand field strength (
 ) for the first time. The structures of the series of complexes, [Ru(NO)L
) for the first time. The structures of the series of complexes, [Ru(NO)L ] (L = Br
] (L = Br , Cl
, Cl , NH
, NH , CN
, CN ), were modeled based on the corresponding single-crystal X-ray coordinates. The comparisons of the geometries and total energies between the different spin states suggested that the singlet spin state of [Ru(II)(NO
), were modeled based on the corresponding single-crystal X-ray coordinates. The comparisons of the geometries and total energies between the different spin states suggested that the singlet spin state of [Ru(II)(NO )L
)L ] complexes were the most stable. The calculated results of both the
] complexes were the most stable. The calculated results of both the  and
and 
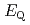 values reproduced the experimental results by reported previously and increased in the order of L = Br
values reproduced the experimental results by reported previously and increased in the order of L = Br , Cl
, Cl , NH
, NH , CN
, CN . Finally, we estimated the ligand field strength (
. Finally, we estimated the ligand field strength (
 ) based on molecular orbitals, assuming C
) based on molecular orbitals, assuming C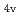 symmetry and showed the increase of
symmetry and showed the increase of 
 values in that order, being consistent with well-known spectrochemical series of ligands. The increase attributes to the strengthening of the abilities of
values in that order, being consistent with well-known spectrochemical series of ligands. The increase attributes to the strengthening of the abilities of  -donor and
-donor and  -acceptor of the L-ligands to the Ru atom, resulting in the increase of the
-acceptor of the L-ligands to the Ru atom, resulting in the increase of the  values.
values.
Sano, Yuichi; Ambai, Hiromu; Takahatake, Yoko
Aichi Shinkurotoron Hikari Senta 2017-Nendo Kokyoto Riyo Seika Hokokusho (Internet), 1 Pages, 2018/00
In order to elucidate the mechanism of corrosion in the reprocessing process and propose a method for suppressing corrosion, the effect of coexisting substances on the chemical form of Ru in nitric acid solution containing seawater components was evaluated. The result of XAFS measurement for Ru showed the structural change around a Ru atom due to the interaction with chloride ion, which will suppress the corrosion promoting action of Ru in nitric acid solution.
Nagai, Takayuki; Kobayashi, Hidekazu; Sasage, Kenichi; Ayame, Yasuo; Okamoto, Yoshihiro; Shiwaku, Hideaki; Yamanaka, Keisuke*; Ota, Toshiaki*
JAEA-Research 2017-005, 54 Pages, 2017/06
Addition of radioactive waste to a borosilicate frit affects the local structures of boron (B) and waste elements in a waste glass. Synchrotron XAFS measurement was applied to investigate the local structural changes by using simulated waste borosilicate glass samples. Following results were obtained by the B K-edge XAFS analysis. It was confirmed that B K-edge XAFS analysis enables us to discriminate sp type boron (BO
type boron (BO ) from sp
) from sp type boron (BO
type boron (BO ). Addition of waste elements to a glass frit increases the percentage of BO
). Addition of waste elements to a glass frit increases the percentage of BO and decreases that of BO
and decreases that of BO . By decreasing the SiO
. By decreasing the SiO /Al
/Al O
O ratio or increasing the (SiO
ratio or increasing the (SiO +B
+B O
O )/Al
)/Al O
O ratio in the glass composition, the BO
ratio in the glass composition, the BO percentage increases and the BO
percentage increases and the BO percentage decreases. Addition of P
percentage decreases. Addition of P O
O decreases the BO
decreases the BO percentage and increases the BO
percentage and increases the BO percentage. Following results were obtained from XAFS measurement of the waste elements. Cerium (Ce) valence is more reduced with the increase of the B
percentage. Following results were obtained from XAFS measurement of the waste elements. Cerium (Ce) valence is more reduced with the increase of the B O
O content. Addition of P
content. Addition of P O
O has a tendency to reduce the Ce valence and to enhance deposition of Zr oxide. Deposition of ruthenium compounds separated from glass phase can not be improved by changing the B
has a tendency to reduce the Ce valence and to enhance deposition of Zr oxide. Deposition of ruthenium compounds separated from glass phase can not be improved by changing the B O
O content. This study was performed as a part of the project, "Improvement of vitrification process of high-level radioactive liquid wastes" on the foundation business of the Agency for Natural Resources and Energy.
content. This study was performed as a part of the project, "Improvement of vitrification process of high-level radioactive liquid wastes" on the foundation business of the Agency for Natural Resources and Energy.
Nagai, Takayuki; Akiyama, Daisuke*; Sato, Nobuaki*; Sasage, Kenichi
Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten Kenkyu Seika Hokokusho (Heisei-28-Nendo) (CD-ROM), 1 Pages, 2017/03
no abstracts in English
Nagai, Takayuki; Sato, Nobuaki*; Sasage, Kenichi
Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten Kenkyu Seika Hokokusho (Heisei-27-Nendo) (CD-ROM), 2 Pages, 2016/03
no abstracts in English
Tashiro, Shinsuke; Amano, Yuki; Yoshida, Kazuo; Yamane, Yuichi; Uchiyama, Gunzo; Abe, Hitoshi
Nihon Genshiryoku Gakkai Wabun Rombunshi, 14(4), p.227 - 234, 2015/12
The release characteristics of Ru from highly active liquid waste (HALW) have been investigated under the condition of accidental evaporation to dryness by boiling of HALW. Using a laboratory-scale apparatus, non-radioactive simulated HALW (s-HALW) was heated with an external heater to dryness to observe the release characteristics of Ru and gaseous nitrogen oxides. As a result, Ru was significantly released between 120 and 300  C of the s-HALW. The cumulative release ratio of Ru was 0.088. It was also found that the partially released amount of Ru against the temperature of the s-HALW had two peaks with one maximal at about 140
C of the s-HALW. The cumulative release ratio of Ru was 0.088. It was also found that the partially released amount of Ru against the temperature of the s-HALW had two peaks with one maximal at about 140  C and maximum at about 240
C and maximum at about 240  C. Referring to the results of the release rate of gaseous nitrogen oxides and the volume of condensate, which was a collection of the mixed vapors of steam and nitric acid released from the s-HALW, we discussed the causes of Ru release around these peaks.
C. Referring to the results of the release rate of gaseous nitrogen oxides and the volume of condensate, which was a collection of the mixed vapors of steam and nitric acid released from the s-HALW, we discussed the causes of Ru release around these peaks.
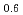 Ru
Ru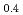 O
O
Yoshii, Kenji; Nakamura, Akio; Mizumaki, Masaichiro*; Tanida, Hajime*; Kawamura, Naomi*; Abe, Hideki*; Ishii, Yoshinobu; Shimojo, Yutaka; Morii, Yukio
Journal of Magnetism and Magnetic Materials, 272-276(Suppl.), p.e609 - e611, 2004/05
Times Cited Count:4 Percentile:25.12(Materials Science, Multidisciplinary)no abstracts in English
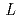
 Sr
Sr CoO
CoO (
(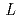 =La, Pr, Nd, Sm, and Eu)
=La, Pr, Nd, Sm, and Eu)Yoshii, Kenji; Abe, Hideki*
Physical Review B, 67(9), p.094408_1 - 094408_8, 2003/03
Times Cited Count:40 Percentile:82.47(Materials Science, Multidisciplinary)no abstracts in English
 NiRuO
NiRuO
Yoshii, Kenji; Abe, Hideki*; Mizumaki, Masaichiro*; Tanida, Hajime*; Kawamura, Naomi*
Journal of Alloys and Compounds, 348(1-2), p.236 - 240, 2003/01
Times Cited Count:15 Percentile:63.77(Chemistry, Physical)no abstracts in English
 ErRuO
ErRuO
Izumiyama, Yuki*; Doi, Yoshihiro*; Wakeshima, Makoto*; Hinatsu, Yukio*; Nakamura, Akio; Ishii, Yoshinobu
Journal of Solid State Chemistry, 169(1), p.125 - 130, 2002/11
Times Cited Count:41 Percentile:81.15(Chemistry, Inorganic & Nuclear)no abstracts in English
Shirasu, Yoshiro; Minato, Kazuo
Journal of Alloys and Compounds, 337(1-2), p.243 - 247, 2002/05
Times Cited Count:3 Percentile:29.91(Chemistry, Physical)no abstracts in English
Shirasu, Yoshiro; Minato, Kazuo
Journal of Alloys and Compounds, 335(1-2), p.224 - 227, 2002/03
Times Cited Count:7 Percentile:47.03(Chemistry, Physical)no abstracts in English

Yoshii, Kenji; Abe, Hideki*
Physica B; Condensed Matter, 312-313(1-4), p.791 - 792, 2002/03
Times Cited Count:6 Percentile:34.98(Physics, Condensed Matter)no abstracts in English
Ueta, Shinzo*
PNC TJ1211 98-001, 824 Pages, 1998/02
None
Uchiyama, Gunzo; Asakura, Toshihide; Hotoku, Shinobu; Watanabe, Makio; *; Fujine, Sachio
JAERI-Research 96-069, 49 Pages, 1997/01
no abstracts in English